

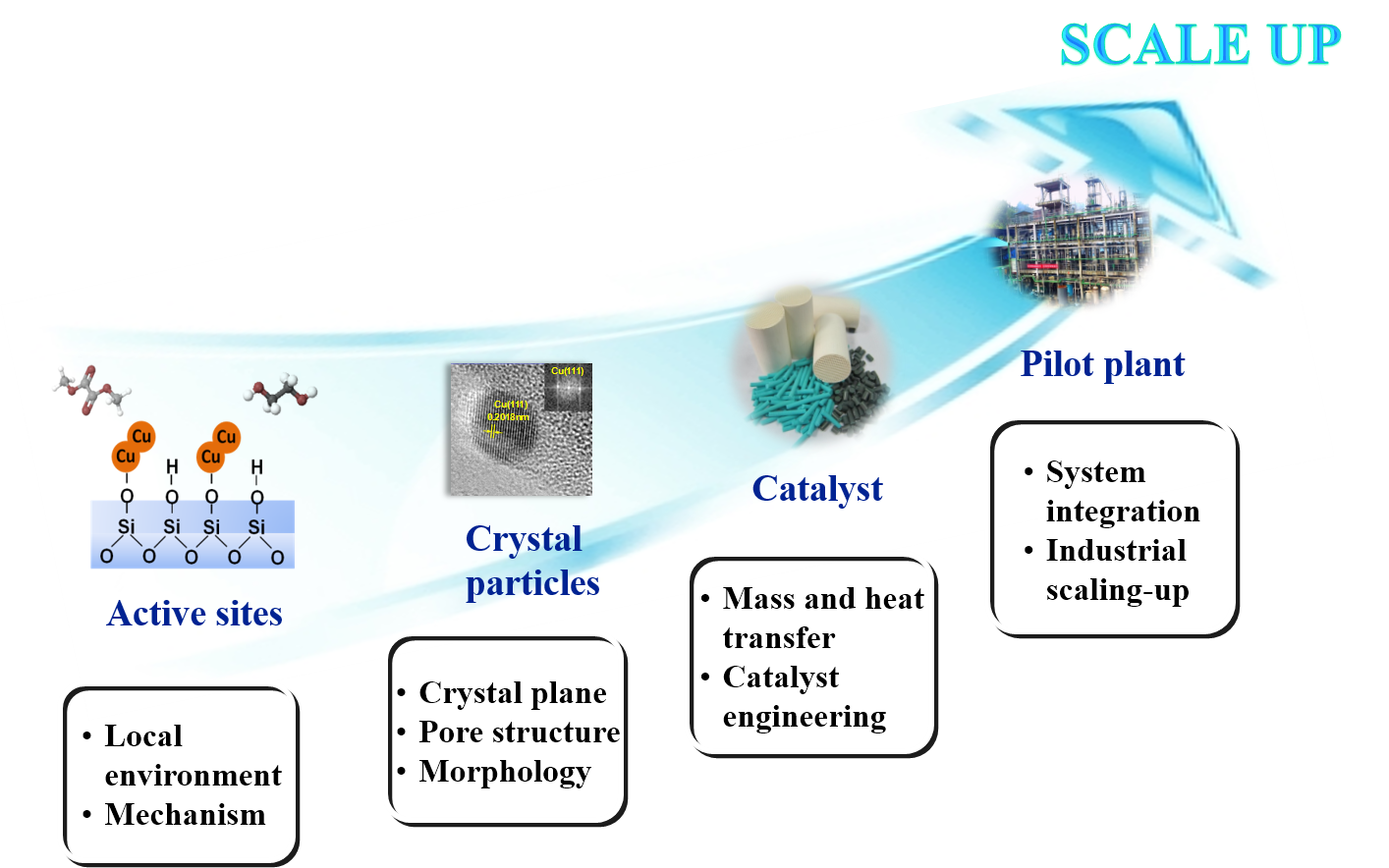
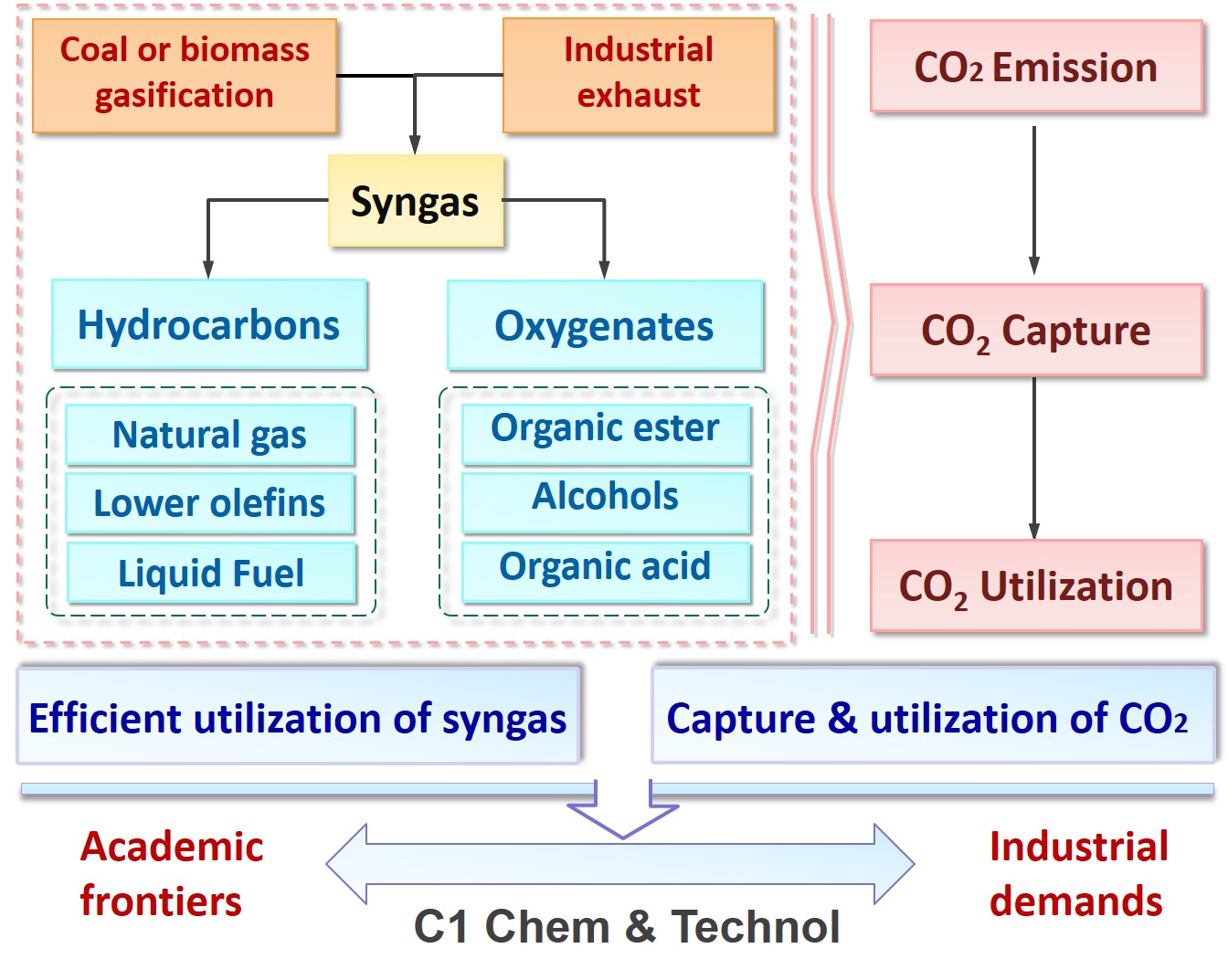
Considering the world oil reserve is depleting and particularly in China, relatively limited crude oil and natural gas but abundant coal resources, it is of significant importance of developing a green technology for conversion of syngas into bulk chemicals. To improve the efficiency of syngas utilization, we focus on developing green technology to produce bulk chemicals from syngas, which include synthesis of oxygenates via carbonylation (alkyl carbonate, oxalate, diphenyl carbonate, ethylene glycol, ethanol, alcohol, etc.), synthesis of low carbon olefins and oil via Fischer-Tropsch technology, sulfur-resistant methanation, etc.
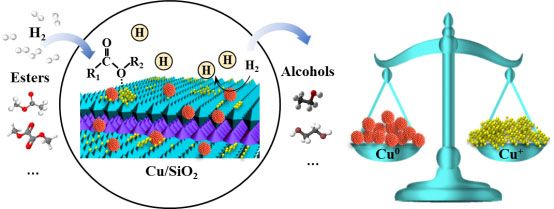
The process consists of two steps which are synthesis of oxalate ester via CO carbonylation and hydrogenation of oxalate into glycol and ethanol. It is green and high efficiency process with adequate utilization of carbon source. The process of oxalate synthesis developed by our group is characterized by a steady self-circulation state including coupling and regeneration steps. And structured monolithic catalysts were developed which reduce the cost by more than 75%. Thus, our technology is highly competitively. In the process of oxalate hydrogenation, we developed Cu/SiO2 catalyst in which the equilibrium of the valence state stability and structural stability was achieved. Based on this, we further propose a novel process which converted syngas into ethanol with high selectivity. Those results were published in JACS, Nature Commun. and other top journals, and they are highlighted on Nature China and JACS. Our key technology for the synthesis of glycol and ethanol and ethanol from syngas is awarded a patent with independent intellectual property rights. A pilot plant with kilotons per year scale which produces ethylene glycol, ethanol from yellow phosphorus exhausted gas has been established in Guizhou, China. And it has successfully passed the on-spot examination organized by experts from China petroleum and chemical industry federation. The pioneered plant is the first process that can produce glycol or ethanol with high selectivity, which is achieved by adjusting the hydrogenation temperature using same equipment and catalyst. It was highlight in "China Chemical News" and other domestic media.
1.2 Conversion of Synthesis gas into Ethanol via Dimethyl Ether carbonylation
A new process for the synthesis of ethanol from syngas was proposed, which consists of synthesis of methyl acetate via dimethyl ether carbonylation and hydrogenation of methyl acetate into ethanol. This process is characterized by high atomic economy, easy separation and recycle of by-product methanol. The developed molecular sieve catalyst for dimethyl ether carbonylation and the copper-based catalyst for methyl acetate hydrogenation are both stable and efficient, which can meet the industrialization needs. We have achieved a pilot plant agreement with industrial companies and are doing works to scale up the process.
Conversion of synthesis gas into oil or low carbon olefins (C2~C4) via Fischer-Tropsch synthesis is the frontier research area in recent years for both domestic and international. The cobalt-based catalyst requires low reaction temperature and high activity, which is suitable for the raw materials with high hydrogen to carbon ratio. The iron-based catalyst has superior steam conversion performance, which eliminate units of water vapor conversion and methanol synthesis of MTO process. Fe-based catalyst can simplify the technology process, and create a novel route from coal to olefins. At the present stage, we mainly focus on the design of Co-based and Fe-based catalyst, the controllable preparation and structure-activity relationship analysis.
1.4 Sulfur-resistant methanationSulfur-resistant methanation is a process of producing natural gas directly using methane as the raw material without steam reforming and desulfurization. This technology integrates the process of sulfur-resistant methanation and water vapor transformation, which can greatly simplify the process flow, reduce capital and operational cost, and improve the utilization of energy. Supported by the national high technology research and development program (863 Program), our group devoted to the development of sulfur-resistant methanation catalyst, process scaled-up, integration and optimization, aiming at forming an independent intellectual property rights of sulfur-resistant methanation which can be used for industrial production, and providing basis for the industrialization of short process of conversion of coal into natural gas.
1.5 Oxidative carbonylation of methanol into alkyl carbonate
The oxidative carbonylation of methanol into alkyl carbonate such as dimethyl carbonate was achieved by the reaction of methanol with CO and O2 or other oxygen carriers. Under the supports of the National Natural Science Foundation of China and Science and Technology Department of Yunnan province, a pilot plant with 300 tons/a dimethyl carbonate production capacity was successfully established using purified yellow phosphorus exhausted gas as the gas source, which realized closed circulation and zero emissions in the whole process. In addition, another pilot project of kilotons/a will also be conducted in the near future.
2. CO2 capture & conversion
Nowadays, the energy structure of human being is dominated by carbon energy. And the "greenhouse effect" caused by the massive emission of CO2 has become a global environmental problem. Hence, reduction the emission of CO2 by CO2 capture and conversion has become a hot topic of scientific research around the world.
CaO is the most widely used adsorbent for CO2 adsorption. Given that the industrial flue gas is a stable, concentrated and long-term CO2 emission source, the most direct and effective way to reduce CO2 emission is recovering CO2 from flue gas. Supported by the National Natural Science Foundation of China and the cooperation project of industry, university and research institute, our group carried out research of developing CaO-based sorbents which can be applied at high temperature. Ca-based adsorbent usually suffers from many challenges, for example, under relatively high adsorption and desorption temperatures the particles tends to aggregation easily. Therefore, to overcome the above-mentioned problems, mixed oxides derived from hydrotalcite and nano-sized spheres of special structure and morphology were developed to improve the adsorbent adsorption performance and recycle stability.
2.2 Synthesis of oxygen-contained compoundsSynthesis of dimethyl carbonate from CO2 and methanol or hydrogenation of CO2 into formic acid are both important ways of CO2 utilization of. Considering the stability of CO2 molecule, it is very important and difficult to rationale design of catalyst to realize the activation of CO2. Carbonylation of CO2 into dimethyl carbonate (DMC) is a thermodynamically unfavorable reaction, thus the key point to improve the yield of DMC is studying the way of force the chemical balance to move forward, which contains addition of water removal agent and the reaction process optimization
2.3 Reforming of dry gasReforming of dry gas (CO2/CH4) into syngas is another way of CO2 utilization. Nickel is the most promising active component for the dry gas reforming catalysts. Nevertheless, the coke deposition and the agglomeration of Ni particle is the key factor to decide whether this reaction can be industrialized or not. Supported by the National Natural Science Foundation of China, the reaction mechanism, structure-activity relationship and deactivation were investigated, in order to develop a stable and efficient nickel based dry gas reforming catalyst which can be used for industrial application.